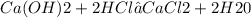Chemistry, 29.09.2019 03:00 Mattisback2285
How many moles of calcium chloride would be produced by the reaction below when 53.2 milliliters of water are produced, if the density of water is 0.987 g/ml? show all steps of your calculations as well as the final answer hcl + ca(oh) 2 → h2o + cacl2

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
How many moles of calcium chloride would be produced by the reaction below when 53.2 milliliters of...
Questions

Computers and Technology, 22.09.2019 21:30

Mathematics, 22.09.2019 21:30



Mathematics, 22.09.2019 21:30



English, 22.09.2019 21:30




Mathematics, 22.09.2019 21:30





Mathematics, 22.09.2019 21:30


Biology, 22.09.2019 21:30