
Chemistry, 02.12.2020 17:00 scottcourtney460
A biochemist carefully measures the molarity of magnesium ion in of cell growth medium to be . Unfortunately, a careless graduate student forgets to cover the container of growth medium and a substantial amount of the solvent evaporates. The volume of the cell growth medium falls to . Calculate the new molarity of magnesium ion in the cell growth medium. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3


You know the right answer?
A biochemist carefully measures the molarity of magnesium ion in of cell growth medium to be . Unfor...
Questions




Mathematics, 28.02.2020 22:00





Mathematics, 28.02.2020 22:00


English, 28.02.2020 22:00


Mathematics, 28.02.2020 22:00


Mathematics, 28.02.2020 22:20

Mathematics, 28.02.2020 22:20


Mathematics, 28.02.2020 22:20


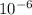 x 47 x
x 47 x 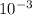 = 4.559 x
= 4.559 x 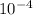 M = 759.83333 uM
M = 759.83333 uM

