
Chemistry, 02.12.2020 05:00 shapeshifter119
"Indicate in each whether the following electron configurations correspond to an atom in its ground state, excited state, or is impossible."
a. 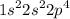
b. 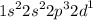
c. 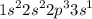
d. 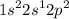
e. 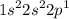
f. 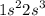
Please, I really need help. I don't know what to do...

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
"Indicate in each whether the following electron configurations correspond to an atom in its ground...
Questions

History, 04.02.2020 01:53

History, 04.02.2020 01:53

Chemistry, 04.02.2020 01:53

History, 04.02.2020 01:53

Physics, 04.02.2020 01:53


Social Studies, 04.02.2020 01:53

History, 04.02.2020 01:53


History, 04.02.2020 01:53


Biology, 04.02.2020 01:53

Mathematics, 04.02.2020 01:53


Mathematics, 04.02.2020 01:53

Social Studies, 04.02.2020 01:53

Mathematics, 04.02.2020 01:53


Mathematics, 04.02.2020 01:53



