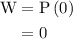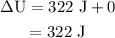Chemistry, 23.12.2019 04:31 pearlkissp1bzl8
Calculate the change in internal energy of the following system: a 100.0-g bar of gold is heated from 25 ∘c to 50 ∘c during which it absorbs 322 j of heat. assume the volume of the gold bar remains constant.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1


Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
Calculate the change in internal energy of the following system: a 100.0-g bar of gold is heated fr...
Questions

English, 18.10.2019 10:00

Spanish, 18.10.2019 10:00

History, 18.10.2019 10:00



Physics, 18.10.2019 10:00





Physics, 18.10.2019 10:00

Computers and Technology, 18.10.2019 10:00


Mathematics, 18.10.2019 10:00

Biology, 18.10.2019 10:00



Mathematics, 18.10.2019 10:00

Health, 18.10.2019 10:00

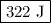
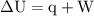 …… (1)
…… (1)
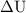 is the change in internal energy of the system.
is the change in internal energy of the system.
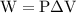 …… (2)
…… (2)
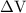 is the change in the volume of the system.
is the change in the volume of the system.