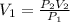Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3


Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
At a pressure of 710 atm, gas has an unknown volume. When the pressure is decreased
to 0.96 atm, th...
Questions



Social Studies, 14.05.2021 02:20


Mathematics, 14.05.2021 02:20



Physics, 14.05.2021 02:20


Mathematics, 14.05.2021 02:20



Spanish, 14.05.2021 02:20

Mathematics, 14.05.2021 02:20



English, 14.05.2021 02:20

Advanced Placement (AP), 14.05.2021 02:20


Chemistry, 14.05.2021 02:20