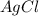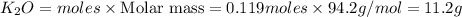Chemistry, 01.12.2020 07:30 ijustneedhelp29
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reagent and what is the theoretical yield of the reaction?
Hint: write the balanced reaction
K - 39.10 g/mol
O - 15.999 g/mol
ANSWER CHOICES:
A.) O2 is limiting, 11.2 g of K2O formed
B.) K is limiting, 14.7 g of K2O formed
C.) K is limiting, 11.2 g of K2O formed
D.) O2 is limiting, 14.7 g of K2O formed
E.) O2 is limiting, 19.2 g of K2O formed

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reag...
Questions




Mathematics, 11.11.2019 17:31


Mathematics, 11.11.2019 17:31



History, 11.11.2019 17:31

English, 11.11.2019 17:31



Mathematics, 11.11.2019 17:31

History, 11.11.2019 17:31

World Languages, 11.11.2019 17:31

Mathematics, 11.11.2019 17:31



History, 11.11.2019 17:31

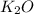 formed
formed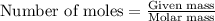
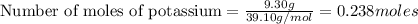
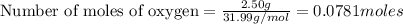
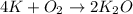
 require 1 mole of
require 1 mole of 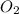
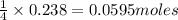 of
of 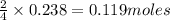 of
of