
Chemistry, 01.12.2020 03:20 christianfielding336
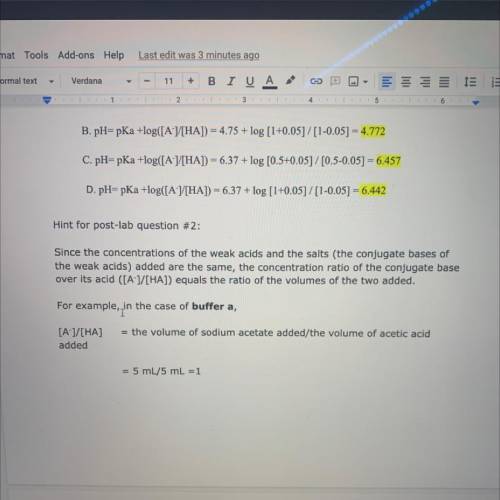
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjugate bases of
the weak acids) added are the same, the concentration ratio of the conjugate base
over its acid ([A-]/[HA]) equals the ratio of the volumes of the two added.
For example, in
in the case of buffer a,
= the volume of sodium acetate added/the volume of acetic acid
[A-]/[HA]
added
= 5 mL/5 mL =1

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjug...
Questions



Social Studies, 15.07.2019 13:00



History, 15.07.2019 13:00

Mathematics, 15.07.2019 13:00

Mathematics, 15.07.2019 13:00


Social Studies, 15.07.2019 13:00

History, 15.07.2019 13:00

Mathematics, 15.07.2019 13:00

Mathematics, 15.07.2019 13:00



Social Studies, 15.07.2019 13:00


History, 15.07.2019 13:00


Spanish, 15.07.2019 13:00



