Electrolysis of water occurs according to the following equation...
2H20 ->2H2+O2
If...

Chemistry, 29.11.2020 23:00 chunkymonkey090
Electrolysis of water occurs according to the following equation...
2H20 ->2H2+O2
If electrolysis is performed with 100% efficiency on 100 grams of water. How many grams of hydrogen gas will be produced?
a. 11 grams
b. 50 grams
c. 4 grams
d. 100 grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Questions

Mathematics, 22.04.2020 13:48

Mathematics, 22.04.2020 13:48



Social Studies, 22.04.2020 13:51


Mathematics, 22.04.2020 13:52


History, 22.04.2020 13:59


Mathematics, 22.04.2020 13:59





Mathematics, 22.04.2020 13:59

Chemistry, 22.04.2020 13:59

Mathematics, 22.04.2020 13:59


Chemistry, 22.04.2020 14:00

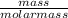
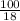 = 5.56mol
= 5.56mol 

