
Chemistry, 29.11.2020 14:40 johndoesnutz4690
Calculate the pH of a solution that is 0.291 M acetic acid and 0.123 M sodium acetate. The Ka of acetic acid is 1.76×10^–5 at 25°C. What is the pH of this mixture at 0°C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
Calculate the pH of a solution that is 0.291 M acetic acid and 0.123 M sodium acetate. The Ka of ace...
Questions




Mathematics, 22.08.2020 01:01

Mathematics, 22.08.2020 01:01




Computers and Technology, 22.08.2020 01:01

History, 22.08.2020 01:01

Mathematics, 22.08.2020 01:01



Mathematics, 22.08.2020 01:01




History, 22.08.2020 01:01


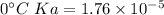
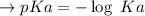
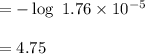
![pH = pKa + \log \frac{[sodium \ acetate]}{[acetic \ acid]}](/tpl/images/0932/3481/1f523.png)
![= 4.75 + \log \frac{[0.123]}{[0.291]}\\\\= 4.75+ \lg(0.422680412)\\\\=4.75-0.373987878\\\\=4.37601212\\\\=4.37](/tpl/images/0932/3481/5061d.png)


