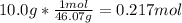Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
How many moles of ethanol C2H6O, are in a 10.0g sample...
Questions

Mathematics, 06.07.2021 14:00

History, 06.07.2021 14:00

Chemistry, 06.07.2021 14:00

Computers and Technology, 06.07.2021 14:00


Social Studies, 06.07.2021 14:00

Physics, 06.07.2021 14:00

Physics, 06.07.2021 14:00

Mathematics, 06.07.2021 14:00



English, 06.07.2021 14:00


Business, 06.07.2021 14:00

Mathematics, 06.07.2021 14:00

Mathematics, 06.07.2021 14:00

Mathematics, 06.07.2021 14:00

English, 06.07.2021 14:00

Chemistry, 06.07.2021 14:00

Mathematics, 06.07.2021 14:00