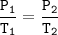Chemistry, 29.11.2020 01:00 skullglitches
Using the same sample of gas (P1 = 605 torr , T1 = 20 ∘C ), we wish to change the pressure to 6050 torr with no accompanying change in volume or amount of gas. What temperature T2, in Celsius, is needed to reach this pressure?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Using the same sample of gas (P1 = 605 torr , T1 = 20 ∘C ), we wish to change the pressure to 6050 t...
Questions

Social Studies, 07.12.2020 18:40



Mathematics, 07.12.2020 18:40

Mathematics, 07.12.2020 18:40



Mathematics, 07.12.2020 18:40


Geography, 07.12.2020 18:40


Mathematics, 07.12.2020 18:40

Mathematics, 07.12.2020 18:40

Mathematics, 07.12.2020 18:40

Chemistry, 07.12.2020 18:40



History, 07.12.2020 18:40

Chemistry, 07.12.2020 18:40

English, 07.12.2020 18:40