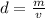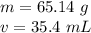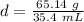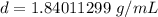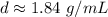Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1
You know the right answer?
Caculate the density of sulfuric acid if 35.4 mL if. The acid weighs 65.14 grams...
Questions

Mathematics, 24.11.2020 20:40

History, 24.11.2020 20:40

Social Studies, 24.11.2020 20:40


Mathematics, 24.11.2020 20:40

Mathematics, 24.11.2020 20:40

English, 24.11.2020 20:40


Mathematics, 24.11.2020 20:40



Social Studies, 24.11.2020 20:40


Mathematics, 24.11.2020 20:40


Biology, 24.11.2020 20:40

Mathematics, 24.11.2020 20:40