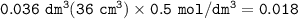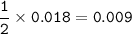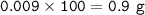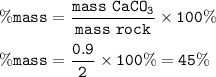Chemistry, 28.11.2020 22:30 nurmukhammada
A piece of rock has a mass of 2.00g. It contains calcium carbonate, but no other
substances. It neutralises exactly 36.0 cm of 0.500 moldmhydrochloric acid.
What is the percentage of calcium carbonate in the 2.00 g piece of rock?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
A piece of rock has a mass of 2.00g. It contains calcium carbonate, but no other
substances. It neu...
Questions

Mathematics, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30

History, 30.09.2019 09:30


Mathematics, 30.09.2019 09:30

English, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30

Health, 30.09.2019 09:30

History, 30.09.2019 09:30



Chemistry, 30.09.2019 09:30

Biology, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30

Biology, 30.09.2019 09:30

Business, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30