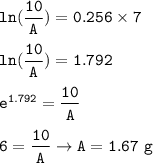Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 18:30
What is the solution to the problem to the correct number of significant figures (102,900/12)+(170•1.27)
Answers: 1
You know the right answer?
3. Gold-198 decays with a half-life of 2.7 days according to the equation:198Au → 0−1β + 198Cd
a. F...
Questions




Mathematics, 23.07.2020 01:01

Biology, 23.07.2020 01:01


English, 23.07.2020 01:01




History, 23.07.2020 01:01

Mathematics, 23.07.2020 01:01




Mathematics, 23.07.2020 01:01




Mathematics, 23.07.2020 01:01

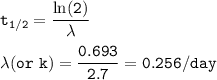
![\tt ln[A]=-kt+ln[Ao]\\\\ln(\dfrac{Ao}{A})=kt](/tpl/images/0931/8187/b434b.png)