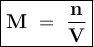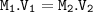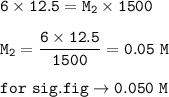Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1


Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1
You know the right answer?
12.5 mL of 6.0 M KCl is diluted to make a 1.5 L solution. The molarity of the dilution solution is _...
Questions

Mathematics, 16.04.2020 16:24


Mathematics, 16.04.2020 16:24

Health, 16.04.2020 16:24

Mathematics, 16.04.2020 16:24

Mathematics, 16.04.2020 16:24


Mathematics, 16.04.2020 16:24






Chemistry, 16.04.2020 16:24

Mathematics, 16.04.2020 16:24

Mathematics, 16.04.2020 16:24



Biology, 16.04.2020 16:24