
Chemistry, 28.11.2020 07:30 xmanavongrove55
25 points , hi! please look at the attachment for the question, I'm having a hard time because all I have to do is solve through those 2 problems but I'm not sure if I solve the fractions first then multiply that result by 100 or what. I asked my teacher which she helped a little but I don't think she understood where I was lost. If you can help I would really appreciate it. Thank you.
This was the set up question:
4. If 100.0g of nitrogen gas (N2) is reacted with 100.0g of hydrogen gas (H2) to form NH3. What is the limiting and excess reactants?
Hint: Convert grams to moles for each reactant and then convert to moles of NH3. You need your balanced equation from answer 1 to determine the mole relationship between each reactant and the product NH3. Use the periodic table to determine the molar mass of all chemical formulas. Fill in the “?” blanks below to show your work.
and in the screenshot it has everything I'm working with and the conclusions I need to draw from it, I can draw the conclusions just fine on my own but I need help solving.
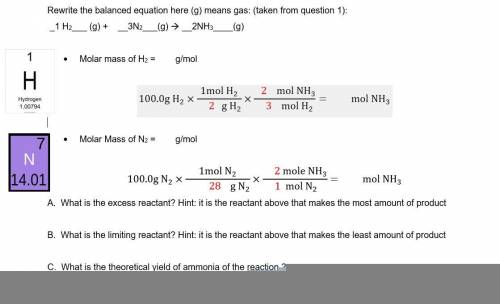

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
25 points , hi! please look at the attachment for the question, I'm having a hard time because all I...
Questions



Mathematics, 20.09.2019 20:30



History, 20.09.2019 20:30



Spanish, 20.09.2019 20:30




Computers and Technology, 20.09.2019 20:30


Mathematics, 20.09.2019 20:30





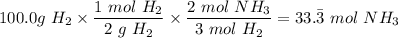
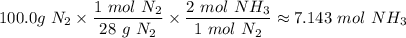
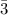 moles of NH₃)
moles of NH₃)

