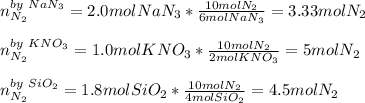Chemistry, 28.11.2020 03:20 isabellemaine
When an airbag expands in a vehicle, sodium azide reacts with potassium nitrate and silicon dioxide, releasing nitrogen gas and sodium potassium silicate (fine glass powder) 6 NaN3+ 2 KNO3 + 4 SiO210 N2+ 2 NaKSiO3+ 2 Na2SiO3 + 02 To conduct a similar reaction, 2.0 mol of NaN3, 1.0 mol of KNO3 and 1.8 mol of SiO2 are added. Which one is the limiting reactant? a) N2 b) O2 c) KNO3 d) SiO2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
When an airbag expands in a vehicle, sodium azide reacts with potassium nitrate and silicon dioxide,...
Questions


History, 08.12.2020 20:20


Mathematics, 08.12.2020 20:20

Mathematics, 08.12.2020 20:20





Mathematics, 08.12.2020 20:20


Mathematics, 08.12.2020 20:20


Social Studies, 08.12.2020 20:20

Social Studies, 08.12.2020 20:20

English, 08.12.2020 20:20

Mathematics, 08.12.2020 20:20

Mathematics, 08.12.2020 20:20


Arts, 08.12.2020 20:20