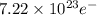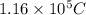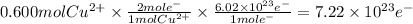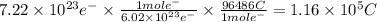Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
You perform an electrochemical reaction in which 0.600 mol of Cu are reduced to solid Cu. How many c...
Questions

Health, 14.10.2019 06:30



Mathematics, 14.10.2019 06:30




Mathematics, 14.10.2019 06:30



Mathematics, 14.10.2019 06:30



History, 14.10.2019 06:30

History, 14.10.2019 06:30

Mathematics, 14.10.2019 06:30

Mathematics, 14.10.2019 06:30

Mathematics, 14.10.2019 06:30