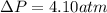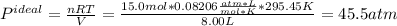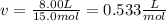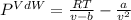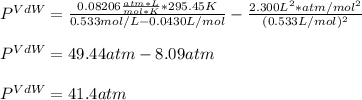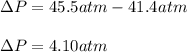Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1

Chemistry, 23.06.2019 10:00
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1

Chemistry, 23.06.2019 11:00
Just on number 2 (all parts), and if you do answer explain in detail
Answers: 3
You know the right answer?
15.0 moles of gas are in a 8.00 L tank at 22.3 ∘C∘C . Calculate the difference in pressure between m...
Questions




Social Studies, 05.02.2020 03:59


Mathematics, 05.02.2020 03:59





French, 05.02.2020 03:59




Mathematics, 05.02.2020 04:00

Mathematics, 05.02.2020 04:00

Physics, 05.02.2020 04:00


Mathematics, 05.02.2020 04:00