
Chemistry, 27.11.2020 14:00 lillysiege
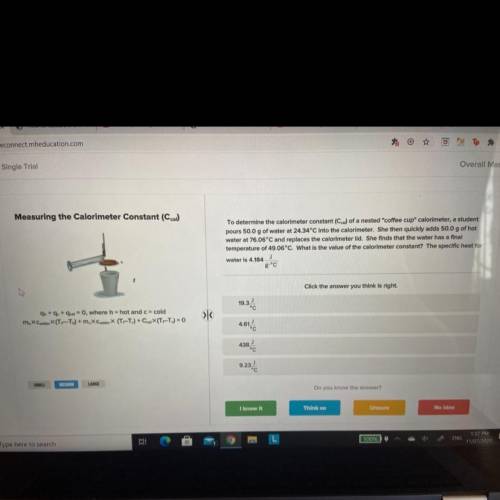
To determine the calorimeter constant (Ccal) of a nested "coffee cup" calorimeter, a student
pours 50.0 g of water at 24.34°C into the calorimeter. She then quickly adds 50.0 g of hot
water at 76.06°C and replaces the calorimeter lid. She finds that the water has a final
temperature of 49.06°C. What is the value of the calorimeter constant? The specific heat for
J
water is 4.184

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
To determine the calorimeter constant (Ccal) of a nested "coffee cup" calorimeter, a student
pours...
Questions

Biology, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00


Mathematics, 28.06.2019 10:00


History, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00


Mathematics, 28.06.2019 10:00



Mathematics, 28.06.2019 10:00


History, 28.06.2019 10:00

History, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00






