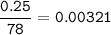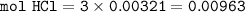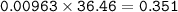Chemistry, 27.11.2020 02:00 carrieaj08
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in antacid tablets neutralizes hydrochloric acid in the stomach. A tablet containing 0.25 g of
aluminum hydroxide is ingested by a patient with 0.88 g of hydrochloric acid in their stomach. Is
this tablet sufficient to neutralize the acid in the patient's stomach? Explain using stoichiometric
calculations. [4 marks]
I

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in ant...
Questions


Mathematics, 24.06.2019 07:00



Business, 24.06.2019 07:00

Biology, 24.06.2019 07:00

Biology, 24.06.2019 07:00

Advanced Placement (AP), 24.06.2019 07:00



Chemistry, 24.06.2019 07:00

History, 24.06.2019 07:00