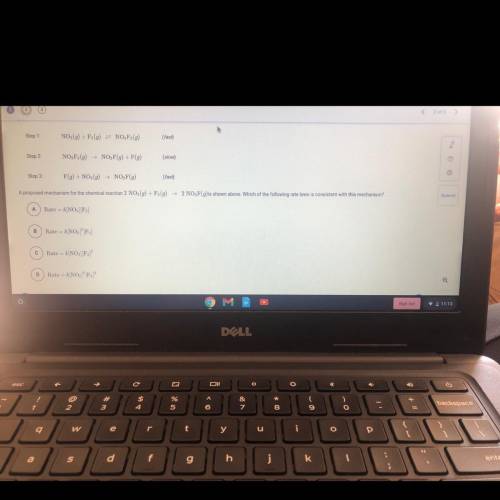
Step 1:
NO2(g) + F2(g) = NO, F2(g)
(fast)
Step 2
NO, F2(9)
NO, F(9) + F(g)<...

Chemistry, 26.11.2020 22:30 ineedhelp2285
Step 1:
NO2(g) + F2(g) = NO, F2(g)
(fast)
Step 2
NO, F2(9)
NO, F(9) + F(g)
(slow)
Step 3:
F(g) + NO (9) NO, F(g)
(fast)
A proposed mechanism for the chemical reaction 2 NO2(g) + F (9)
2 NO, F(g)is shown above. Which of the following rate laws is consistent with this mechanism?
A
Rate = k/NO2][F]
B
Rate = k[NO][F)
С
Rate -- k[NO] F2)
D
Rate
k[NO, F,

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1


Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Questions

Biology, 14.10.2021 01:00




English, 14.10.2021 01:00


Mathematics, 14.10.2021 01:00


Mathematics, 14.10.2021 01:00

Spanish, 14.10.2021 01:00

History, 14.10.2021 01:10

Biology, 14.10.2021 01:10

Computers and Technology, 14.10.2021 01:10

History, 14.10.2021 01:10

History, 14.10.2021 01:10



Mathematics, 14.10.2021 01:10

English, 14.10.2021 01:10

Mathematics, 14.10.2021 01:10



