
Chemistry, 26.11.2020 21:20 Anshuman2002
Ammonia is formed from the reaction of nitrogen and hydrogen according to the equation:
N2(g) + 3H2(g) 2NH3(g)
What is the maximum number of moles of ammonia that can be formed from the reaction of 27 moles of hydrogen?
A
41
B
27
18
D
9

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Ammonia is formed from the reaction of nitrogen and hydrogen according to the equation:
N2(g) + 3H2...
Questions


Mathematics, 13.05.2021 01:00



World Languages, 13.05.2021 01:00




Mathematics, 13.05.2021 01:00

Chemistry, 13.05.2021 01:00


Mathematics, 13.05.2021 01:00





Biology, 13.05.2021 01:00



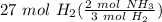 = 18 mol NH₃
= 18 mol NH₃

