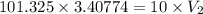Chemistry, 26.11.2020 04:00 Soccermen1021
A weather balloon with a volume of 3.40774 L
is released from Earth’s surface at sea level.
What volume will the balloon occupy at an
altitude of 20.0 km, where the air pressure is
10 kPa?
Answer in units of L.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2
You know the right answer?
A weather balloon with a volume of 3.40774 L
is released from Earth’s surface at sea level.
Questions




Physics, 18.05.2021 01:50



Mathematics, 18.05.2021 01:50

Advanced Placement (AP), 18.05.2021 01:50



History, 18.05.2021 01:50


Mathematics, 18.05.2021 01:50


Mathematics, 18.05.2021 01:50

Biology, 18.05.2021 01:50

Mathematics, 18.05.2021 01:50



Mathematics, 18.05.2021 01:50

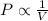 (At constant temperature and number of moles)
(At constant temperature and number of moles)
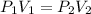
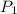 = initial pressure of gas = 101.325 kPa ( at sea level)
= initial pressure of gas = 101.325 kPa ( at sea level)
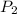 = final pressure of gas = 10 kPa
= final pressure of gas = 10 kPa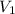 = initial volume of gas = 3.40774 L
= initial volume of gas = 3.40774 L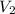 = final volume of gas = ?
= final volume of gas = ?