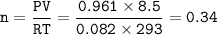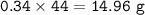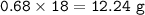Chemistry, 26.11.2020 03:00 kell22wolf
If 8.50 L of natural gas, which is essentially methane (CH4), undergoes complete combustion at 730 mm Hg and 20 degrees C, how many grams of each product are formed?
Grams of CO2=
Grams of H2O=

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 11:20
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2

Chemistry, 23.06.2019 14:00
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature.
Answers: 1
You know the right answer?
If 8.50 L of natural gas, which is essentially methane (CH4), undergoes complete combustion at 730 m...
Questions






Mathematics, 19.05.2021 20:10

Mathematics, 19.05.2021 20:10





Mathematics, 19.05.2021 20:10


Mathematics, 19.05.2021 20:10

English, 19.05.2021 20:10

Mathematics, 19.05.2021 20:10



Computers and Technology, 19.05.2021 20:10