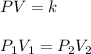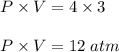Chemistry, 25.11.2020 08:20 applereams
A given sample of a gas has a volume of 3 liters at a
pressure of 4 atmospheres. If temperature remains
constant and the pressure is changed to 6
atmospheres, the P x V product will equal
A). 9 B) 12 C) 18 D) 24

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
A given sample of a gas has a volume of 3 liters at a
pressure of 4 atmospheres. If temperature rem...
Questions

English, 17.07.2019 05:30

Social Studies, 17.07.2019 05:30


English, 17.07.2019 05:30

Chemistry, 17.07.2019 05:30

Biology, 17.07.2019 05:30

Social Studies, 17.07.2019 05:30


Mathematics, 17.07.2019 05:30


Mathematics, 17.07.2019 05:30

History, 17.07.2019 05:30

Mathematics, 17.07.2019 05:30

Computers and Technology, 17.07.2019 05:30

Social Studies, 17.07.2019 05:30


History, 17.07.2019 05:30


History, 17.07.2019 05:30

Social Studies, 17.07.2019 05:30

 product of this given sample of gas is equal to: B) 12 atm.
product of this given sample of gas is equal to: B) 12 atm.