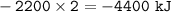For this reaction, C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O, the ∆H is –2200 kJ. If two moles of C3H8(g) reacted with excess oxygen, what would be true?
A) 4400 kj of heat released into surroundings
B) 4400 kj of heat absorbed by system
C) 1100 kJ heat released into surroundings
D) 1100 kJ heat absorbed by system

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
For this reaction, C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O, the ∆H is –2200 kJ. If two moles of C3H8(g)...
Questions

History, 28.08.2020 17:01


Social Studies, 28.08.2020 17:01

History, 28.08.2020 17:01

Physics, 28.08.2020 17:01

Computers and Technology, 28.08.2020 17:01



Physics, 28.08.2020 17:01









History, 28.08.2020 17:01


Physics, 28.08.2020 17:01