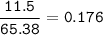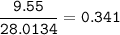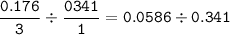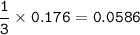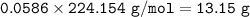Chemistry, 24.11.2020 19:10 natalia9573
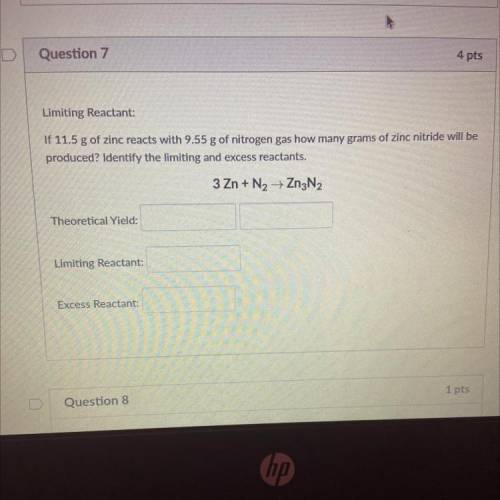
Limiting Reactant:
If 11.5 g of zinc reacts with 9.55 g of nitrogen gas how many grams of zinc nitride will be
produced? Identify the limiting and excess reactants.
3 Zn + N2 + Zn3N2
Theoretical Yield:
Limiting Reactant:
Excess Reactant:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1


Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
Limiting Reactant:
If 11.5 g of zinc reacts with 9.55 g of nitrogen gas how many grams of zinc nitr...
Questions

Mathematics, 29.10.2020 21:30

Mathematics, 29.10.2020 21:30

History, 29.10.2020 21:30


Engineering, 29.10.2020 21:30

Mathematics, 29.10.2020 21:30






Mathematics, 29.10.2020 21:30

Mathematics, 29.10.2020 21:30



Mathematics, 29.10.2020 21:30

Mathematics, 29.10.2020 21:30


Mathematics, 29.10.2020 21:30