
Chemistry, 24.11.2020 14:00 gjvyverman
II. Ionic Equations
8. Write the complete ionic and net ionic equations for the reaction below:
2 AgNO3(aq) + CaCl2(aq) → 2 AgCl + Ca(NO3)2
Complete ionic:
Net ionic:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

You know the right answer?
II. Ionic Equations
8. Write the complete ionic and net ionic equations for the reaction below:
Questions

Mathematics, 18.03.2021 20:50


Arts, 18.03.2021 20:50



Mathematics, 18.03.2021 20:50



Mathematics, 18.03.2021 20:50


Biology, 18.03.2021 20:50

History, 18.03.2021 20:50

Mathematics, 18.03.2021 20:50






Mathematics, 18.03.2021 20:50

Mathematics, 18.03.2021 20:50

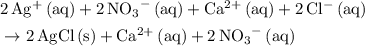 .
.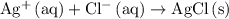 .
. 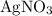 ,
, 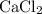 , and
, and 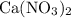 . These three salts will exist as ions:
. These three salts will exist as ions: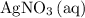 formula unit will exist as one
formula unit will exist as one 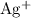 ion and one
ion and one 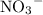 ion. Each
ion. Each 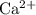 ion and two
ion and two 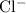 ions (note the subscript in the formula
ions (note the subscript in the formula 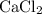 .) Each
.) Each 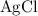 is generally insoluble in water. This salt will not form ions.
is generally insoluble in water. This salt will not form ions.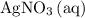 in the original equation is two,
in the original equation is two, 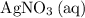 alone should correspond to two
alone should correspond to two 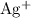 ions and two
ions and two 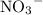 ions.
ions. 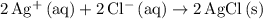 .
.

