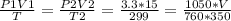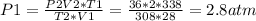2) A gas takes up a volume of 15 liters, has a pressure of 3.3 atm, and a temperature of
299 K. If I raise the temperature to 350 K and lower the pressure to 1050 mmHg, what is the
new volume of the gas?
3) A gas that has a volume of 28 liters, a temperature of 65 °C, and an unknown pressure
has its volume increased to 36 liters and its temperature decreased to 35 °C. If I measure the
pressure after the change to be 2.0 atm, what was the original pressure of the gas?
work too pls

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
2) A gas takes up a volume of 15 liters, has a pressure of 3.3 atm, and a temperature of
299 K. If...
Questions

Social Studies, 03.12.2020 14:00




Mathematics, 03.12.2020 14:00

History, 03.12.2020 14:00

Mathematics, 03.12.2020 14:00

Mathematics, 03.12.2020 14:00


Physics, 03.12.2020 14:00

History, 03.12.2020 14:00



Mathematics, 03.12.2020 14:00


Business, 03.12.2020 14:00


Mathematics, 03.12.2020 14:00


Chemistry, 03.12.2020 14:00