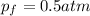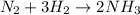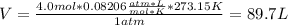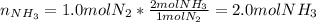Chemistry, 24.11.2020 05:00 aleilyg2005
If 1.0 mol of N2 and 3.0 mol of H2 in a closed container initially at STP react completely in the reaction shown below, then the final pressure in the flask will be atm at 273 K.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
If 1.0 mol of N2 and 3.0 mol of H2 in a closed container initially at STP react completely in the re...
Questions

Mathematics, 26.08.2019 17:00



Mathematics, 26.08.2019 17:00

Health, 26.08.2019 17:00

Computers and Technology, 26.08.2019 17:00


History, 26.08.2019 17:00


English, 26.08.2019 17:00

Mathematics, 26.08.2019 17:00

Biology, 26.08.2019 17:00

Mathematics, 26.08.2019 17:00



Mathematics, 26.08.2019 17:00


Geography, 26.08.2019 17:00