

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3


Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1
You know the right answer?
. Laughing gas is used by dentists as an anesthetic. If a 10.0 L tank of laughing gas contains 1.26...
Questions






Mathematics, 28.01.2020 18:10



History, 28.01.2020 18:40



Geography, 28.01.2020 18:40

English, 28.01.2020 18:40


History, 28.01.2020 18:40

Mathematics, 28.01.2020 18:40



Mathematics, 28.01.2020 18:40

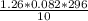 = 3.058atm
= 3.058atm

