
Chemistry, 23.11.2020 20:50 arangoaiden71
Calculate the change of enthalpy for the reaction CH4 (g) + NH3 (g) --> HCN (g) +3H2 (g) from the following reactions:
Reaction 1: N2 (g) + 3H2 (g) --> 2NH3 (g) Change in enthalpy: -91.8 kJ/mol
Reaction 2: C (s, graphite) + 2H2 (g) --> CH4 (g) Change in enthalply: -74.9 kJ/mol
Reaction 3: H2 (g) + 2C (s, graphite) + N2 (g) --> 2HCN (g) Change in enthalpy: +270.3 kJ/mol
Include the following:
The numerical answer with correct units.
State which reactions, if any, you had to "Flip".
State which reactions you had to multiply, if any, to get the correct amount of the compound.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
Calculate the change of enthalpy for the reaction CH4 (g) + NH3 (g) --> HCN (g) +3H2 (g) from the...
Questions

Social Studies, 18.12.2019 07:31



Mathematics, 18.12.2019 07:31

Physics, 18.12.2019 07:31

English, 18.12.2019 07:31






Biology, 18.12.2019 07:31


Biology, 18.12.2019 07:31


Physics, 18.12.2019 07:31

Chemistry, 18.12.2019 07:31


Social Studies, 18.12.2019 07:31

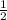 in order to have it cancel out with the other reactions. For the third reaction, we'd want to we'd want to switch the products/reactants and multiply the coefficients by
in order to have it cancel out with the other reactions. For the third reaction, we'd want to we'd want to switch the products/reactants and multiply the coefficients by 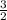 H₂ --> NH₃ (ΔH=-45.9)
H₂ --> NH₃ (ΔH=-45.9)

