
Chemistry, 21.11.2020 22:20 maxy7347go
The density of ethanol, C 2H 5OH, is 0.789 g/mL. How many milliliters of ethanol are needed to produce 25.0 g of CO 2 according to the following chemical equation? C 2H 5OH( l) + 3 O 2( g) → 2 CO 2( g) + 3 H 2O( l)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
The density of ethanol, C 2H 5OH, is 0.789 g/mL. How many milliliters of ethanol are needed to produ...
Questions


Mathematics, 24.11.2019 08:31




Mathematics, 24.11.2019 08:31


Engineering, 24.11.2019 08:31


Computers and Technology, 24.11.2019 08:31

History, 24.11.2019 08:31

Biology, 24.11.2019 08:31


Mathematics, 24.11.2019 08:31

Mathematics, 24.11.2019 08:31

Health, 24.11.2019 08:31

Mathematics, 24.11.2019 08:31



Mathematics, 24.11.2019 08:31

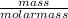
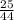 = 0.57mol
= 0.57mol 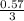 = 0.19mole of ethanol
= 0.19mole of ethanol 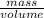
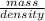
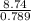 = 11.1mL
= 11.1mL

