Measuring rates of reaction
RoR2a
Aim: To measure the rate at which calcium carbonate reacts...

Chemistry, 21.11.2020 15:20 aschool2000
Measuring rates of reaction
RoR2a
Aim: To measure the rate at which calcium carbonate reacts with hydrochloric acid by
measuring the volume of carbon dioxide produced at intervals during the reaction.
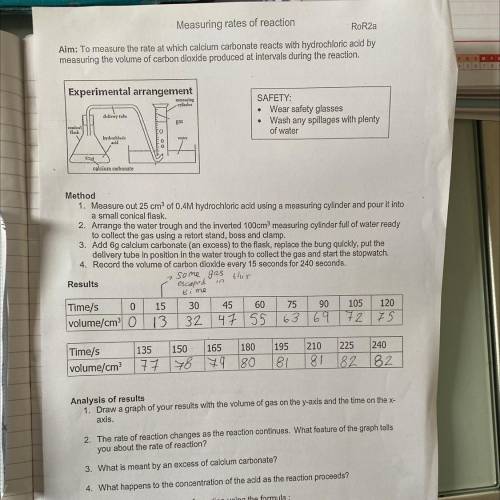
Experimental arrangement
measuring
cylinder
delivery tube
SAFETY:
Wear safety glasses
Wash any spillages with plenty
of water
gas
conical
flask
hydrochloric
adid
water
calcium carbonate
Method
1. Measure out 25 cm3 of 0.4M hydrochloric acid using a measuring cylinder and pour it into
a small conical flask.
2. Arrange the water trough and the inverted 100cmº measuring cylinder full of water ready
llatba
start stond bocsandalam

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:40
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0172 m solution. if the ph of the resulting solution is 2.39 at 20 °c, determine the pka for the acid.
Answers: 1


Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

You know the right answer?
Questions

Mathematics, 16.11.2020 03:20

Mathematics, 16.11.2020 03:20


Mathematics, 16.11.2020 03:20


Mathematics, 16.11.2020 03:20

Mathematics, 16.11.2020 03:20

English, 16.11.2020 03:20


English, 16.11.2020 03:20

Mathematics, 16.11.2020 03:20


Mathematics, 16.11.2020 03:20



Biology, 16.11.2020 03:20

Computers and Technology, 16.11.2020 03:20





