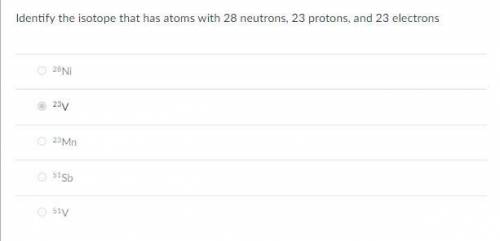
Identify the isotope that has atoms with 28 neutrons, 23 protons, and 23 electrons
A. ^28 Ni
...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1


You know the right answer?
Questions

History, 04.10.2020 18:01

Biology, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01



Social Studies, 04.10.2020 18:01


Mathematics, 04.10.2020 18:01

Chemistry, 04.10.2020 18:01

Computers and Technology, 04.10.2020 18:01

Medicine, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01

English, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01

History, 04.10.2020 18:01

English, 04.10.2020 18:01

Mathematics, 04.10.2020 18:01

World Languages, 04.10.2020 18:01