
Chemistry, 20.11.2020 21:50 estefaniapenalo
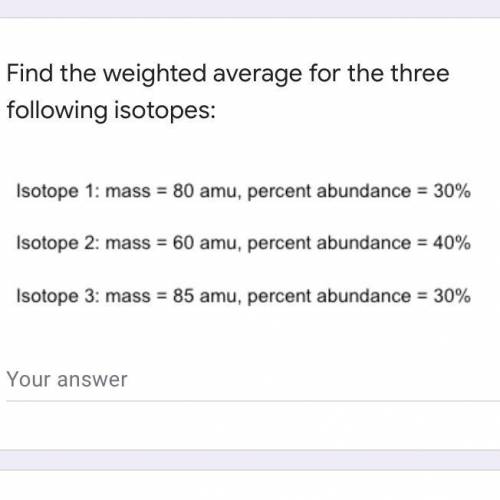
Find the weighted average for the three following isotopes: Isotope 1: mass = 80 amu, percent abundance dance=30\% Isotope 2: mass = 60 amu, percent 1dance=40\% Isotope 3: mass = 85 amu, percent abundance 1dance=30\%

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3


Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Find the weighted average for the three following isotopes: Isotope 1: mass = 80 amu, percent abunda...
Questions



History, 16.07.2019 17:30

History, 16.07.2019 17:30

History, 16.07.2019 17:30

History, 16.07.2019 17:30

Chemistry, 16.07.2019 17:30

Biology, 16.07.2019 17:30

Biology, 16.07.2019 17:30

English, 16.07.2019 17:30

English, 16.07.2019 17:30

English, 16.07.2019 17:30






Social Studies, 16.07.2019 17:30


Business, 16.07.2019 17:30



