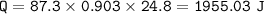Chemistry, 20.11.2020 19:30 marykatherine
How many Joules of energy are needed to raise 87.3 grams of aluminum from 22,5 C to 47.3. C. The specific heat of aluminum is 0.903 V&C. Joules

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
How many Joules of energy are needed to raise 87.3 grams of aluminum from 22,5 C to 47.3. C. The spe...
Questions

History, 09.06.2021 21:20

Mathematics, 09.06.2021 21:20





Mathematics, 09.06.2021 21:20


Mathematics, 09.06.2021 21:30



Mathematics, 09.06.2021 21:30


Mathematics, 09.06.2021 21:30


Arts, 09.06.2021 21:30

Business, 09.06.2021 21:30


Health, 09.06.2021 21:30

Mathematics, 09.06.2021 21:30