Chemistry, 20.11.2020 17:00 queenpaige2015
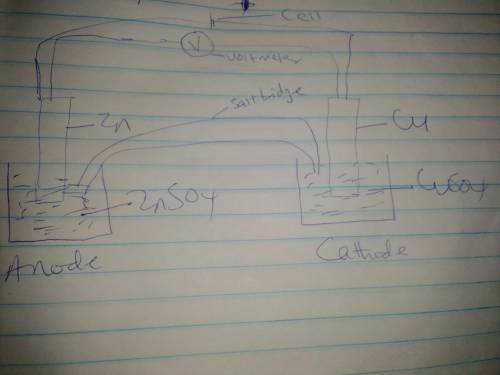
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76 V Cu^2+ + 2 e^> Cu E°cell = 0.34 V 1. Predict the standard potential of the cell at 298 K. 2. What is the minimum voltage that should be applied to the standard electrolytic cell found in question to cause zn2+ to be reduced to Zn?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76...
Questions

Chemistry, 01.04.2020 21:29



Mathematics, 01.04.2020 21:29

Mathematics, 01.04.2020 21:29




English, 01.04.2020 21:29

Mathematics, 01.04.2020 21:29


Mathematics, 01.04.2020 21:29

Mathematics, 01.04.2020 21:29

Mathematics, 01.04.2020 21:29

Mathematics, 01.04.2020 21:29

Mathematics, 01.04.2020 21:29


Mathematics, 01.04.2020 21:29

Mathematics, 01.04.2020 21:29

Health, 01.04.2020 21:29