
Chemistry, 20.11.2020 17:00 tsimonej12
What is the molarity of an aqueous solution that contains 0.072 g C2H6O2 per gram of solution? The density of the solution is 1.40 g/mL.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
What is the molarity of an aqueous solution that contains 0.072 g C2H6O2 per gram of solution? The d...
Questions




Mathematics, 20.02.2020 17:12



Chemistry, 20.02.2020 17:13






English, 20.02.2020 17:14





History, 20.02.2020 17:14


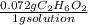 =0.1008 g C₂H₆O₂
=0.1008 g C₂H₆O₂


