
Chemistry, 20.11.2020 14:00 biancaceja755
Methane (CH4) is the main component of marsh gas. Heating methane in the presence of sulfur produces carbon disulfide and hydrogen sulfide as the only products. (a) Write the balanced chemical equation for the reaction of methane and sulfur. (Use the lowest possible coefficients. Include states of matter under SATP conditions in your answer.)(b) Calculate the theoretical yield of carbon disulfide when g of methane is reacted with an equal mass of sulfur.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

You know the right answer?
Methane (CH4) is the main component of marsh gas. Heating methane in the presence of sulfur produces...
Questions

Mathematics, 23.09.2020 15:01

Mathematics, 23.09.2020 15:01



Mathematics, 23.09.2020 15:01



History, 23.09.2020 15:01


Mathematics, 23.09.2020 15:01



Mathematics, 23.09.2020 15:01

Mathematics, 23.09.2020 15:01



History, 23.09.2020 15:01

Mathematics, 23.09.2020 15:01


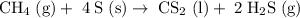
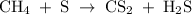
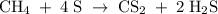
 molecular weight
molecular weight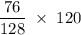 grams Carbon disulfide
grams Carbon disulfide

