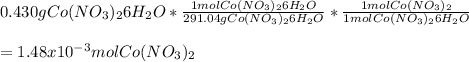Chemistry, 20.11.2020 14:00 meramera50
If you mass 0.430 g of Co(NO3)2 6H2O and dissolve it in water, what further information would you need to calculate:.
a. the number of moles of Co(NO3)2 in the solution?
b. the molarity of the solution

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2


Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
If you mass 0.430 g of Co(NO3)2 6H2O and dissolve it in water, what further information would you ne...
Questions


Mathematics, 22.08.2019 00:30




History, 22.08.2019 00:30

Social Studies, 22.08.2019 00:30



Mathematics, 22.08.2019 00:30

English, 22.08.2019 00:30


Mathematics, 22.08.2019 00:30


Mathematics, 22.08.2019 00:30

Social Studies, 22.08.2019 00:30


Mathematics, 22.08.2019 00:30

Mathematics, 22.08.2019 00:30