
Chemistry, 20.11.2020 14:00 eboniwiley
A piece of iron is heated to 95.0 celsius and then placed in an insulated vessel containing 250. grams of water at 25.0 celsius. when the system comes to equilibrium the temperature of the system is 35.0 celsius. what is the mass in g of the iron? assume no heat is lost to the surroundingsSpecific heat/J. g^-1 K^-1iron 0.450water 4.184 (A) 4.48 g (B) 41.7 g (C) 387 g (D) 612 g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
A piece of iron is heated to 95.0 celsius and then placed in an insulated vessel containing 250. gra...
Questions







Physics, 12.06.2020 21:57





Mathematics, 12.06.2020 21:57

Mathematics, 12.06.2020 21:57







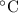 × (308 K - 298 K)
× (308 K - 298 K)


