

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2
You know the right answer?
Look at the balanced equation below:
C2H6O + 3 O2 → 2 CO2 + 3 H2O
If you produced 11.5...
If you produced 11.5...
Questions


Physics, 07.04.2020 00:34


Health, 07.04.2020 00:34

Mathematics, 07.04.2020 00:34


Computers and Technology, 07.04.2020 00:34

Mathematics, 07.04.2020 00:34







Social Studies, 07.04.2020 00:35



English, 07.04.2020 00:35


History, 07.04.2020 00:35

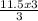 = 11.5moles of oxygen gas
= 11.5moles of oxygen gas

