
Chemistry, 20.11.2020 07:20 tdluong157
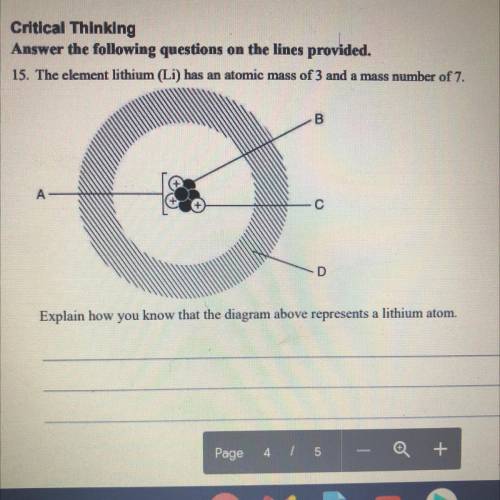
15. The element lithium (Li) has an atomic mass of 3 and a mass number of 7. Explain how you know that the diagram above represents a lithium atom.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
15. The element lithium (Li) has an atomic mass of 3 and a mass number of 7.
Explain how you know...
Questions

Mathematics, 23.11.2021 14:10

Social Studies, 23.11.2021 14:10


Social Studies, 23.11.2021 14:10

Mathematics, 23.11.2021 14:10

Biology, 23.11.2021 14:10

Mathematics, 23.11.2021 14:10

Engineering, 23.11.2021 14:10

English, 23.11.2021 14:10



Computers and Technology, 23.11.2021 14:10


Mathematics, 23.11.2021 14:10

Mathematics, 23.11.2021 14:10

Mathematics, 23.11.2021 14:10

Biology, 23.11.2021 14:10

Mathematics, 23.11.2021 14:10

English, 23.11.2021 14:10



