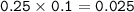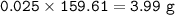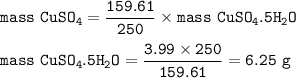Chemistry, 19.11.2020 20:10 Braxtonw875
Approximately what mass of CuSO4*5H20 (250 g mol^-1) is required to prepare 250 mL of 0.10 M copper(ll)
sulfate solution?
A) 4.0 g
B
6.2 g
С
34 g
D
85 g
E
140 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1


Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
Approximately what mass of CuSO4*5H20 (250 g mol^-1) is required to prepare 250 mL of 0.10 M copper(...
Questions

Mathematics, 29.12.2019 15:31


Mathematics, 29.12.2019 15:31




Mathematics, 29.12.2019 15:31

English, 29.12.2019 15:31



Biology, 29.12.2019 15:31



Mathematics, 29.12.2019 15:31



Chemistry, 29.12.2019 15:31

Physics, 29.12.2019 15:31


History, 29.12.2019 15:31