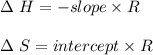A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the solubility of your salt. B. Would this initial evaporation affect the calculated solubility of your salt at each subsequent experimental saturation temperature, or just the initial temperature? Explain.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the so...
Questions



Mathematics, 03.09.2020 16:01







Mathematics, 03.09.2020 16:01

History, 03.09.2020 16:01

History, 03.09.2020 16:01

Mathematics, 03.09.2020 16:01

Mathematics, 03.09.2020 16:01

English, 03.09.2020 16:01

History, 03.09.2020 16:01

Mathematics, 03.09.2020 16:01



Mathematics, 03.09.2020 16:01

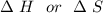 are calculated by
are calculated by 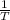 v/s lnKsp
v/s lnKsp