
Chemistry, 19.11.2020 19:00 makaylaf9479
The equilibrium constant Kc for the reaction below is 0.00584 at a certain temperature. Br2(g) ⇌ 2Br(g) If the initial concentrations are [Br2] = 0.0345 M and [Br] = 0.0416 M, calculate the concentrations of these species at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
The equilibrium constant Kc for the reaction below is 0.00584 at a certain temperature. Br2(g) ⇌ 2Br...
Questions

Mathematics, 06.05.2020 05:08


Chemistry, 06.05.2020 05:08

Mathematics, 06.05.2020 05:08





English, 06.05.2020 05:08



Mathematics, 06.05.2020 05:08


Mathematics, 06.05.2020 05:08


History, 06.05.2020 05:08


Chemistry, 06.05.2020 05:08

Chemistry, 06.05.2020 05:08

![Q_C = \dfrac{[Br]^2}{[Br_2]} = \dfrac{(0.0416)^2}{(0.0345)}= 0.05016](/tpl/images/0913/4933/a957d.png)
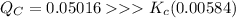
![K_c = \dfrac{[Br]^2}{[Br_2]} = \dfrac{(0.0416-2x)^2}{(0.0345+x)} = 0.00584](/tpl/images/0913/4933/fba5c.png)


