
Chemistry, 17.10.2019 03:30 amberchule9681
Iron(ii) sulfide reacts with hydrochloric acid according to the reaction:
fes(s)+2hcl(aq)→fecl2(s)+h2s(g)
a reaction mixture initially contains 0.240 mol fes and 0.646 mol hcl.
what amount (in moles) of the excess reactant is left?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Iron(ii) sulfide reacts with hydrochloric acid according to the reaction:
fes(s)+2hcl(aq)→fe...
fes(s)+2hcl(aq)→fe...
Questions

Biology, 24.06.2019 03:30

Chemistry, 24.06.2019 03:30



Advanced Placement (AP), 24.06.2019 03:30















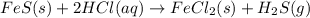
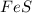 react with 2 mole of
react with 2 mole of 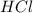
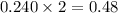 moles of
moles of 

