Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
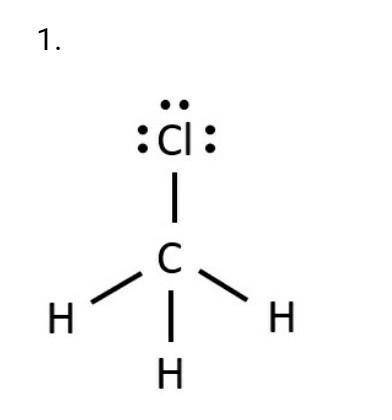
CAN U GUYS HELP ME THIS WAS DUE LAST WEEK AND IM FAILING CHEM PLS HELP
a. What is the molecular geo...
Questions

Mathematics, 10.07.2019 04:50


Social Studies, 10.07.2019 04:50

Social Studies, 10.07.2019 04:50

Social Studies, 10.07.2019 04:50





Social Studies, 10.07.2019 04:50


Social Studies, 10.07.2019 04:50

Social Studies, 10.07.2019 04:50

Biology, 10.07.2019 04:50

History, 10.07.2019 04:50


Mathematics, 10.07.2019 04:50

Mathematics, 10.07.2019 04:50

Biology, 10.07.2019 04:50